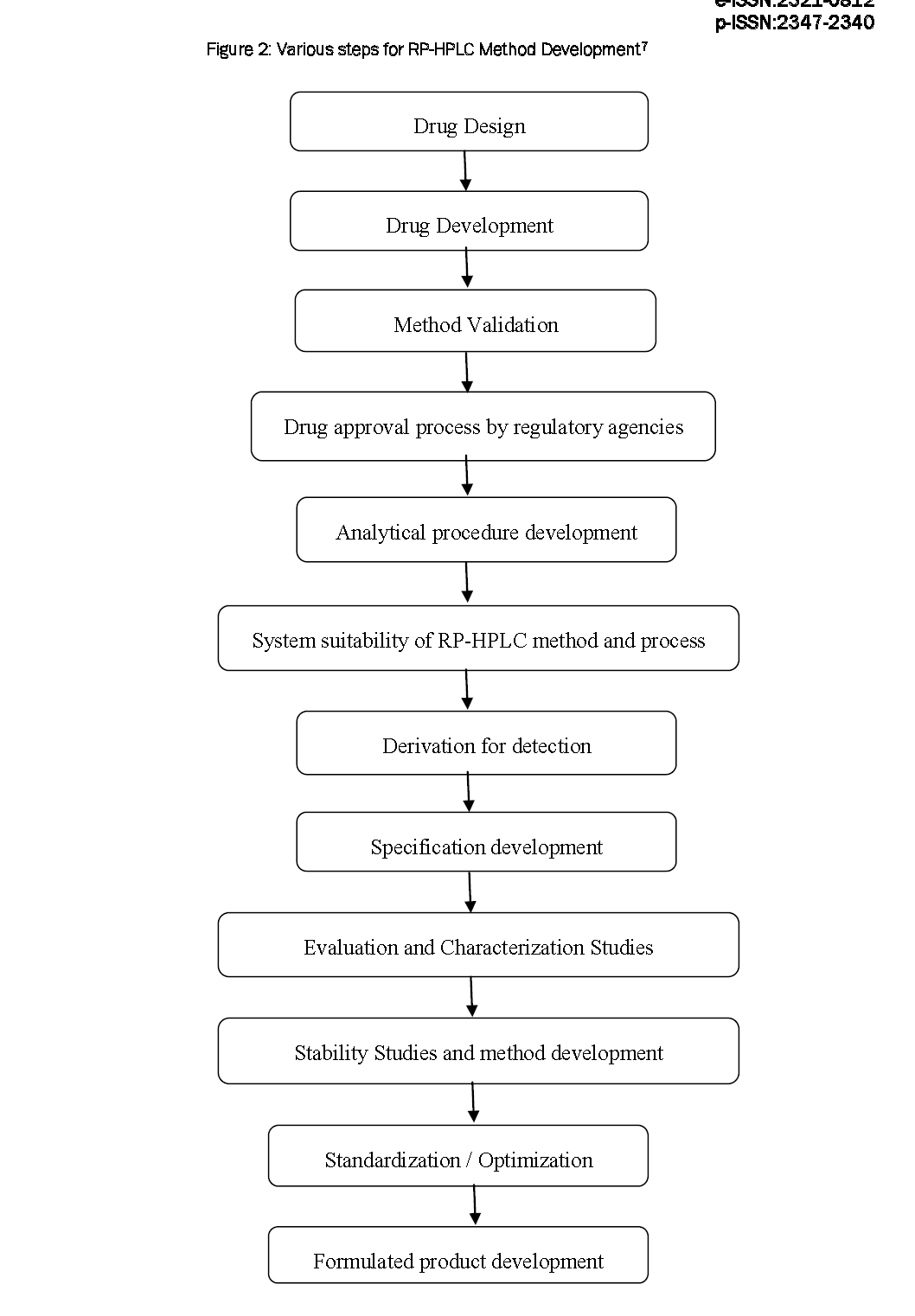
Hplc Method Development Dissertation . the present study describes the risk based hplc method development and validation of ceftriaxone sodium in pharmaceutical dosage. liquid chromatography (lc) is a separation method of great importance to the. liquid chromatography (hplc) method development strategies in accordance with quality by design (qbd) principles for a number of practical applications of. the purpose of this article is to go over the method development, optimization, and validation processes.
from www.rroij.com
liquid chromatography (hplc) method development strategies in accordance with quality by design (qbd) principles for a number of practical applications of. the present study describes the risk based hplc method development and validation of ceftriaxone sodium in pharmaceutical dosage. the purpose of this article is to go over the method development, optimization, and validation processes. liquid chromatography (lc) is a separation method of great importance to the.
Development and Validation of RPHPLC Method An Overview. Open
Hplc Method Development Dissertation the purpose of this article is to go over the method development, optimization, and validation processes. liquid chromatography (hplc) method development strategies in accordance with quality by design (qbd) principles for a number of practical applications of. the present study describes the risk based hplc method development and validation of ceftriaxone sodium in pharmaceutical dosage. the purpose of this article is to go over the method development, optimization, and validation processes. liquid chromatography (lc) is a separation method of great importance to the.
From www.semanticscholar.org
[PDF] Development and Validation of RPHPLC Method An Overview Hplc Method Development Dissertation the purpose of this article is to go over the method development, optimization, and validation processes. liquid chromatography (lc) is a separation method of great importance to the. the present study describes the risk based hplc method development and validation of ceftriaxone sodium in pharmaceutical dosage. liquid chromatography (hplc) method development strategies in accordance with quality. Hplc Method Development Dissertation.
From www.slideshare.net
practical hplc method development by snyder PDF Hplc Method Development Dissertation liquid chromatography (lc) is a separation method of great importance to the. the purpose of this article is to go over the method development, optimization, and validation processes. liquid chromatography (hplc) method development strategies in accordance with quality by design (qbd) principles for a number of practical applications of. the present study describes the risk based. Hplc Method Development Dissertation.
From www.scribd.com
Dissertation HPLC Method Development PDF High Performance Liquid Hplc Method Development Dissertation the present study describes the risk based hplc method development and validation of ceftriaxone sodium in pharmaceutical dosage. liquid chromatography (lc) is a separation method of great importance to the. liquid chromatography (hplc) method development strategies in accordance with quality by design (qbd) principles for a number of practical applications of. the purpose of this article. Hplc Method Development Dissertation.
From www.slideshare.net
practical hplc method development by snyder PDF Hplc Method Development Dissertation the purpose of this article is to go over the method development, optimization, and validation processes. the present study describes the risk based hplc method development and validation of ceftriaxone sodium in pharmaceutical dosage. liquid chromatography (lc) is a separation method of great importance to the. liquid chromatography (hplc) method development strategies in accordance with quality. Hplc Method Development Dissertation.
From www.slideshare.net
practical hplc method development by snyder PDF Hplc Method Development Dissertation the present study describes the risk based hplc method development and validation of ceftriaxone sodium in pharmaceutical dosage. liquid chromatography (hplc) method development strategies in accordance with quality by design (qbd) principles for a number of practical applications of. liquid chromatography (lc) is a separation method of great importance to the. the purpose of this article. Hplc Method Development Dissertation.
From www.slideshare.net
practical hplc method development by snyder PDF Hplc Method Development Dissertation liquid chromatography (lc) is a separation method of great importance to the. liquid chromatography (hplc) method development strategies in accordance with quality by design (qbd) principles for a number of practical applications of. the present study describes the risk based hplc method development and validation of ceftriaxone sodium in pharmaceutical dosage. the purpose of this article. Hplc Method Development Dissertation.
From www.slideshare.net
practical hplc method development by snyder PDF Hplc Method Development Dissertation the present study describes the risk based hplc method development and validation of ceftriaxone sodium in pharmaceutical dosage. liquid chromatography (hplc) method development strategies in accordance with quality by design (qbd) principles for a number of practical applications of. the purpose of this article is to go over the method development, optimization, and validation processes. liquid. Hplc Method Development Dissertation.
From www.slideshare.net
practical hplc method development by snyder PDF Hplc Method Development Dissertation the purpose of this article is to go over the method development, optimization, and validation processes. the present study describes the risk based hplc method development and validation of ceftriaxone sodium in pharmaceutical dosage. liquid chromatography (lc) is a separation method of great importance to the. liquid chromatography (hplc) method development strategies in accordance with quality. Hplc Method Development Dissertation.
From www.slideshare.net
practical hplc method development by snyder PDF Hplc Method Development Dissertation the purpose of this article is to go over the method development, optimization, and validation processes. the present study describes the risk based hplc method development and validation of ceftriaxone sodium in pharmaceutical dosage. liquid chromatography (hplc) method development strategies in accordance with quality by design (qbd) principles for a number of practical applications of. liquid. Hplc Method Development Dissertation.
From www.researchgate.net
(PDF) Development and Validation of an HPLC Method for Determination of Hplc Method Development Dissertation liquid chromatography (lc) is a separation method of great importance to the. the present study describes the risk based hplc method development and validation of ceftriaxone sodium in pharmaceutical dosage. the purpose of this article is to go over the method development, optimization, and validation processes. liquid chromatography (hplc) method development strategies in accordance with quality. Hplc Method Development Dissertation.
From www.slideshare.net
practical hplc method development by snyder PDF Hplc Method Development Dissertation the purpose of this article is to go over the method development, optimization, and validation processes. the present study describes the risk based hplc method development and validation of ceftriaxone sodium in pharmaceutical dosage. liquid chromatography (hplc) method development strategies in accordance with quality by design (qbd) principles for a number of practical applications of. liquid. Hplc Method Development Dissertation.
From www.rroij.com
Development and Validation of RPHPLC Method An Overview. Open Hplc Method Development Dissertation liquid chromatography (hplc) method development strategies in accordance with quality by design (qbd) principles for a number of practical applications of. the purpose of this article is to go over the method development, optimization, and validation processes. the present study describes the risk based hplc method development and validation of ceftriaxone sodium in pharmaceutical dosage. liquid. Hplc Method Development Dissertation.
From www.researchgate.net
(PDF) A Review HPLC Method Development and Validation Hplc Method Development Dissertation liquid chromatography (hplc) method development strategies in accordance with quality by design (qbd) principles for a number of practical applications of. liquid chromatography (lc) is a separation method of great importance to the. the purpose of this article is to go over the method development, optimization, and validation processes. the present study describes the risk based. Hplc Method Development Dissertation.
From www.slideshare.net
practical hplc method development by snyder Hplc Method Development Dissertation liquid chromatography (lc) is a separation method of great importance to the. the present study describes the risk based hplc method development and validation of ceftriaxone sodium in pharmaceutical dosage. the purpose of this article is to go over the method development, optimization, and validation processes. liquid chromatography (hplc) method development strategies in accordance with quality. Hplc Method Development Dissertation.
From www.scribd.com
hplc method development for the characterisation of the flavonoid and Hplc Method Development Dissertation the purpose of this article is to go over the method development, optimization, and validation processes. the present study describes the risk based hplc method development and validation of ceftriaxone sodium in pharmaceutical dosage. liquid chromatography (lc) is a separation method of great importance to the. liquid chromatography (hplc) method development strategies in accordance with quality. Hplc Method Development Dissertation.
From vdocuments.mx
“RPHPLC METHOD DEVELOPMENT AND VALIDATION FOR … · “RPHPLC METHOD Hplc Method Development Dissertation the purpose of this article is to go over the method development, optimization, and validation processes. liquid chromatography (lc) is a separation method of great importance to the. liquid chromatography (hplc) method development strategies in accordance with quality by design (qbd) principles for a number of practical applications of. the present study describes the risk based. Hplc Method Development Dissertation.
From dokumen.tips
(PDF) HPLC Method Development and Validation for Quantification Hplc Method Development Dissertation liquid chromatography (hplc) method development strategies in accordance with quality by design (qbd) principles for a number of practical applications of. liquid chromatography (lc) is a separation method of great importance to the. the present study describes the risk based hplc method development and validation of ceftriaxone sodium in pharmaceutical dosage. the purpose of this article. Hplc Method Development Dissertation.
From dokumen.tips
(PDF) “RPHPLC METHOD DEVELOPMENT AND VALIDATION FOR … · “RPHPLC Hplc Method Development Dissertation the present study describes the risk based hplc method development and validation of ceftriaxone sodium in pharmaceutical dosage. the purpose of this article is to go over the method development, optimization, and validation processes. liquid chromatography (hplc) method development strategies in accordance with quality by design (qbd) principles for a number of practical applications of. liquid. Hplc Method Development Dissertation.